The pulse oximetry test is a non-invasive test that measures how well the heart is pumping oxygen through the body. A pulse oximeter is a small, clip-like device that utilizes the simple spectrophotometry principles of absorption to monitor the percentage of hemoglobin that is oxygen-saturated.
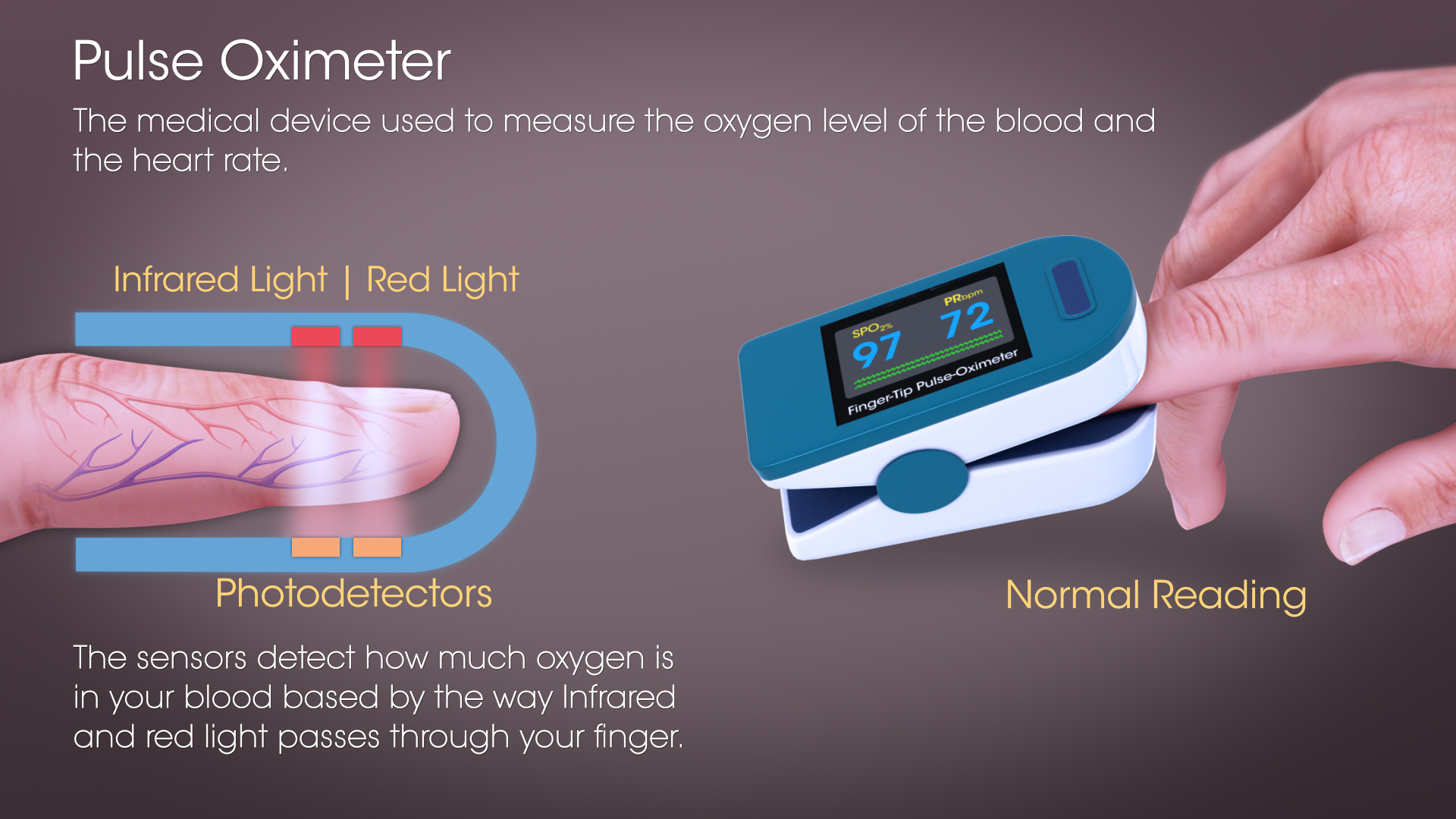
Mechanism
The relative absorption of red light (absorbed by deoxygenated blood) and infrared light (absorbed by oxygenated blood) during a systole, tells the oxygen saturation level of the arterial blood. A well pumping heart maintains the oxygen saturation level.
For a healthy individual, oxygen saturation should be above 95%, although in those with long-standing respiratory disease or cyanotic congenital heart disease, it may be lower, corresponding to disease severity. 92% indicates potential hypoxemia. The oxyhemoglobin dissociation curve becomes sharply steep below about 90%, reflecting the more rapid desaturation that occurs with diminishing oxygen partial pressure. This is the minimum oxygen saturation level needed to keep the body cells healthy; consistent instances of lowered oxygen saturation levels may be damaging.
History and Use
Pulse oximeters are helpful for anesthesiologists as they are used for continuously assessing hypoxemia during anesthesia to diagnose obstructive sleep apnea.
Pulse oximetry is also a cheaper, easier, less painful and more accurate replacement for blood gas analysis in many clinics. It is used to monitor individuals suffering from conditions like COPD, asthma, pneumonia, lung cancer, anemia, heart attack, in which the blood oxygen levels go down.
There has been growing interest in the use of fetal pulse oximetry in combination with routine cardiotocography monitoring, although its use does not reduce the operative delivery rate. The safety limits for oxygen saturations are higher and narrower (95-97%) in neonates compared to those for adults.
Besides, some other common uses of pulse oximetry include:
To check the performance of a new medicine for lung disorders.
To evaluate how helpful a ventilator is.
To determine how effective supplemental oxygen therapy is, especially when treatment is new.
To estimate how much physical activity a person can tolerate.
To assess the lungs of a newborn.
Sources of error
Pulse oximetry is typically a fairly accurate test. It consistently provides results within a 2% plus or minus range. For example, a reading of 82% indicates an oxygen saturation level between 80-84%. However, there may be fluctuations due to the reasons given below:
It’s not possible to differentiate between different forms of hemoglobin using a pulse oximetry procedure. Carboxyhemoglobin is registered as 90% oxygenated hemoglobin and 10% desaturated hemoglobin, thereby causing an overestimation of true saturation levels.
Significant venous pulsation as such occurs in tricuspid incompetence and venous congestion.
Environmental interference such as excessive movement or vibration, and even high level of ambient light, including infrared heat lamps.
Cold hands, nail polish may cause false readings.
Intravascular dyes, such as methylthioninium chloride, may also temporarily falsely reduce saturation readings.
Conclusion
Pulse oximetry has become an important diagnostic tool and safety indicator. It brings particularly vital information into the Operation Room, The Intensive Care Unit, and to Neonatal Care, and more generally, is used as a safety indicator for many inpatients. It is important that pulse oximetry be available in treatment facilities.
In most high-income countries where pulse oximetry is universal across the perioperative process, anesthesia has become very safe: mortality rates directly linked to anesthesia are typically less than 1 in 50,000 procedures. However, in low-income countries these rates are at least ten times higher.
Upgraded to the highest level of recommendation in the updated 2010 International Standards for a Safe Practice of anesthesia, developed and endorsed by the World Federation of Societies of Anesthesiology, pulse oximetry is essentially mandatory – and dangerously scarce in low-resource countries.
Disclaimer: The information in no way constitutes, or should be construed as medical advice. Nor is the above article an endorsement of any research findings discussed in the article an endorsement for any of the source publications.

How Medical Devices undergo FDA Regulation?
Until only a few years back, innovative new drugs and medical devices became available in other countries much before they became accessible to the U.S. citizens. The reason was FDA’s approval process, which thoroughly checks not only product safety but effectiveness. Read More..








